
Patient support services
ServierONE offers patients helpful resources and tools for navigating treatment care, costs, and education throughout their journey.
ServierONE Patient Support Services for TIBSOVO® (ivosidenib tablets) includes:
To receive more information
Monday-Friday, 8 AM to 6 PM ET
Ordering and distribution network
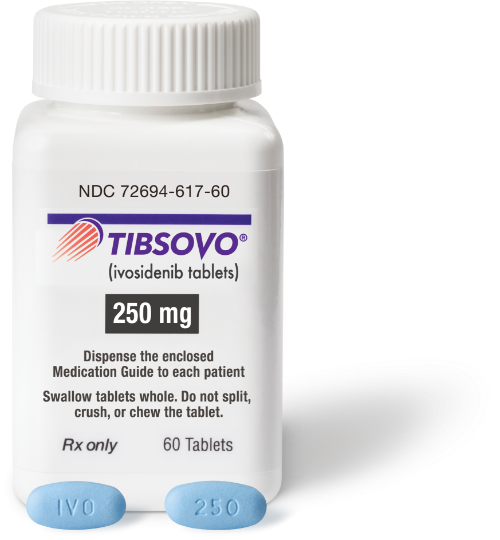
National Drug Code (NDC)
| NDCs |
10-digit code: 72694-617-60 11-digit code: 72694-0617-60 |
| Dosage strength | 250 mg/tablet | Description | 250-mg tablet: Blue oval-shaped film-coated tablet debossed “IVO” on one side and “250” on the other side |
The red zero converts the 10-digit NDC to the 11-digit NDC. Some payers may require each NDC to be listed on the claim. Payer requirements regarding the use of NDCs may vary. Electronic data exchange generally requires use of the 11-digit NDC.
Product information
How TIBSOVO is supplied: 250-mg tablets, supplied in 60-count bottles (30-day supply) with a desiccant canister
Storage: Store at 20 to 25 ºC (68 to 77 ºF)
Distribution network for TIBSOVO
TIBSOVO is only available through our specialty distributors and network specialty pharmacies.
Specialty distributors
McKesson Specialty Health
Cardinal Health Specialty Pharmaceutical Distribution (US)
Cardinal Health (Puerto Rico)
ASD Healthcare
Oncology Supply
Network specialty pharmacies
Biologics by McKesson
Experts agree: Justin M. Watts, MD and Harry Erba, MD, PhD talk about the impact of TIBSOVO + azacitidine in mIDH1 AML
Addressing the Burden of mIDH1 Acute Myeloid Leukemia: An Expert Review of Clinical Data
See Dr. Justin M. Watts, a chief researcher and leukemia expert with the University of Miami and Sylvester Comprehensive Care Center, explain the importance of molecular testing to inform prescribing options. Dr. Watts notes how mIDH1 is a driver mutation in up to 14% of AML patients.
TIBSOVO® + Azacitidine: A Combination Treatment Option for Newly Diagnosed, IC-Ineligible mIDH1 AML
Watch Dr. Harry Erba, a nationally recognized hematology and oncology expert at Duke University in Durham, North Carolina, discuss the key unmet needs among patients with IC-ineligible AML, review the importance of molecular testing, and evaluate the clinical efficacy and safety profile that supports the use of TIBSOVO + azacitidine in adult patients with newly diagnosed, IC-ineligible mIDH1 AML.
